The self-stabilizing cervical fusion cage is an intervertebral spacer made of radiolucent PEEK (polyether–ether ketone) with integrated pure-tantalum X‑ray markers.
It is inserted between two cervical vertebrae and secured with titanium-alloy screws, restoring disc height and normal cervical lordosis. The cage’s unique design uses a distraction–compression stabilization mechanism (originally described by Bagby) to achieve immediate segmental stability: by distracting the disc space and then locking the implant, opposing forces are generated that resist rotation and shear, providing early fixation to aid fusion.
In practice, the cage reconstructs the anterior spinal column after discectomy, bears axial loads in place of the removed disc, and maintains tension in the annulus and ligamentous structures. While it provides instant stability, ultimate fusion depends on bony ingrowth through and around the implant.
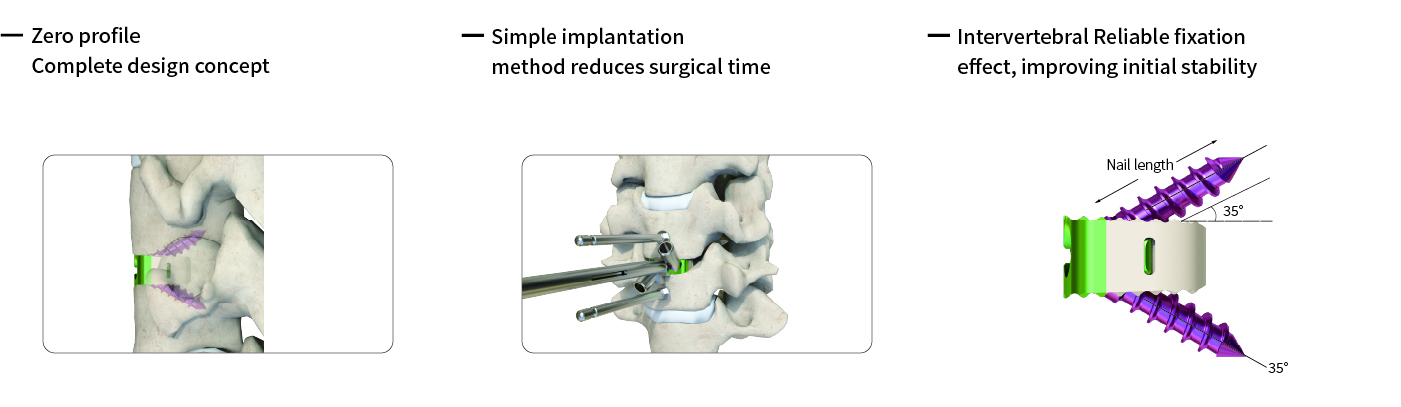
● Immediate Stability and Load Sharing: The cage’s hollow-core PEEK body can be packed with bone graft while its overall geometry restores disc height and sagittal alignment. Serrated (saw‑toothed) teeth on the superior and inferior surfaces bite into the vertebral endplates, increasing friction to prevent migration.
● Zero‑Profile (No Protruding Plate): The implant sits entirely within the disc space (zero-profile design), eliminating the need for an additional anterior plate. This minimizes contact with soft tissues and may reduce adjacent‑level degeneration and postoperative dysphagia.
● Radiographic Markers for Visualization: Pure tantalum pins (“developing needles”) are embedded in the cage (made visible on X‑ray/CT) to facilitate accurate intraoperative and postoperative assessment of implant positioning.The PEEK body itself is radiolucent, making the tantalum markers the only visible component on imaging.
● Bone‑Like Elastic Modulus: PEEK has an elastic modulus close to cancellous bone, which helps distribute stress more evenly and reduces stress-shielding of adjacent vertebrae. This “bone‑friendly” elasticity encourages bone growth and fusion, while the cage’s high fatigue strength supports long-term load-bearing.
● Biocompatibility and Imaging Compatibility: The PEEK material is chemically inert and biocompatible, with a well‑documented safety profile. It is also MRI‑compatible (no metal artifact in MRI) and corrosion-free. The titanium screws and tantalum markers ensure mechanical durability and visibility without sacrificing imaging clarity.
● Secure Locking Mechanism: The cage is locked into place via screws into a small fixation block (also TC4 titanium) on its side. A snap-ring anti‑backout mechanism between the screw and fixation block prevents screw loosening after implantation.
● Broad Sizing Options: The system offers multiple cage footprints and heights (various length × width × height combinations) to match patient anatomy. Surgeons can select the optimal size for each case.
● Streamlined Procedure: As a stand-alone, zero-profile device, no separate titanium plate is needed. Fewer screws are required (typically two) and there is no need to contour or apply a plating system, which can reduce operative time. Minimally invasive insertion via a small anterior approach lowers soft-tissue trauma and may decrease wound complications.
● Reduced Complications: The low-profile, flush fit within the disc space avoids anterior hardware protrusion. This can significantly reduce the risk of plate-related issues (implant loosening, screw back-out, plate breakage) and postoperative dysphagia often seen with traditional plate-and-cage constructs. Avoiding a long plate may also lessen adjacent-segment ossification.
● Enhanced Fusion Potential: By closely matching bone stiffness, the PEEK cage reduces stress-shielding and encourages load-sharing by the graft/bone. The hollow graft chamber allows packing ample autograft or allograft. Together with serrated endplates and immediate mechanical stability, this creates an optimal environment for solid arthrodesis.
● Clear Imaging: Radiolucent PEEK and the tantalum marker pins allow unobstructed visualization of fusion progress on X-ray or CT. This helps clinicians monitor healing without interference from the implant.










